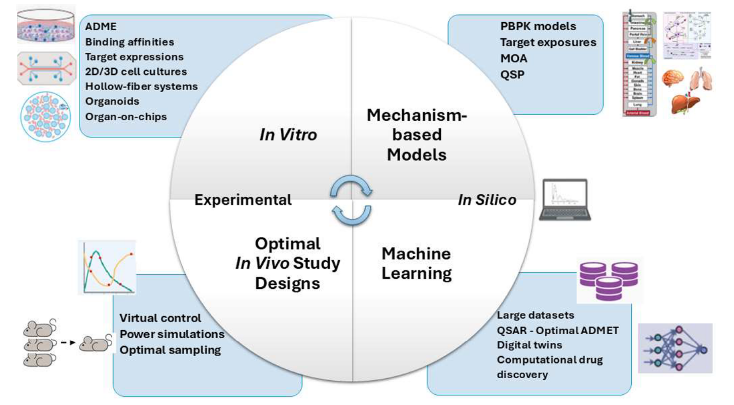
NAMLink accelerates the shift towards reduction, replacement, and refinement (3Rs) of animal use in drug discovery and development by uniting biology, computational modeling, and technology through the integration of new approach methodologies (NAMs).
What are NAMs and why we need to adopt them?
To date, over 90% of drug candidates still fail in clinical development—largely because traditional animal models fall short in predicting human outcomes. As new therapeutic modalities like antibodies, cell and gene therapies emerge, this translation gap is widening.
Human-relevant New Approach Methodologies (NAMs)—including advanced in vitro systems, in silico mechanistic models, and AI-driven analytics—offer a more predictive, ethical, and efficient alternative. They not only reduce reliance on animal testing but also enable earlier, data-informed decisions that improve the success rate of clinical translation.
Backed by growing regulatory support through initiatives such as the FDA Modernization Act 2.0 and EMA Directive 2010/63/EU, the time to integrate NAMs into drug development is now—transforming preclinical science into a more human-relevant, responsible, and forward-looking enterprise.