PBPK Models
Physiologically based pharmacokinetic (PBPK) modeling is a computational approach that predicts how a drug moves through the body—how it is absorbed, distributed, metabolized, and eliminated. Unlike traditional methods that rely heavily on animal studies, PBPK models can use lab-based (in vitro) and physicochemical property data to simulate drug behavior in humans before any in vivo testing. This makes PBPK a powerful New Approach Methodology (NAM) for reducing animal use in early drug development.
How PBPK Can Help Reduce Animal Studies?
PBPK models can be built “from the bottom up,” using only in vitro measurements and known biological information. As shown in case studies discussed Mehta et al., 2025, these bottom-up models have successfully predicted real in vivo drug levels for several small molecules and biologics. Also, as discussed, in a priori workflow, PBPK models can be built early using only in vitro and existing biological knowledge to predict drug behavior before any animal studies. By generating these predictions upfront, PBPK helps design only the minimal, most targeted in vivo studies—reducing the number of animals needed while improving study efficiency.
Mechanistic Insights Not Feasible From Animal Data Alone
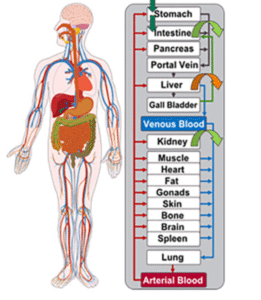
PBPK can also predict drug levels in specific organs—such as the brain, tumors, or other hard-to-sample tissues. Studies have shown PBPK models accurately estimating brain and tumor exposure where direct measurement in animals would be invasive, limited, or impossible. This helps researchers understand drug behavior while avoiding difficult or high-burden animal procedures.
When Combined With Advanced In Vitro Systems
PBPK can become even more useful when paired with modern in vitro technologies such as organ-on-chip systems. These systems generate human-relevant data that PBPK can translate into whole-body predictions. For example, combining liver-on-chip data with PBPK has accurately predicted human drug clearance and variability across different individuals [Aravindakshan et al., 2025].
PBPK: A Robust Tool NAM for Preclinical Development
Together, these features make PBPK one of the most practical and ready-to-use tools in the NAM space. It supports smarter, smaller, and more ethical preclinical study designs while improving confidence in human-relevant predictions.
Learn more about PBPK models using the following resources (more to come):
A Priori NAM Workflow for Preclinical Development
An a priori NAM workflow uses non-animal methods at the very start of preclinical development to generate human-relevant insights before any in vivo studies. By predicting drug behavior upfront, this approach helps reduce the number and scope of animal experiments needed later.
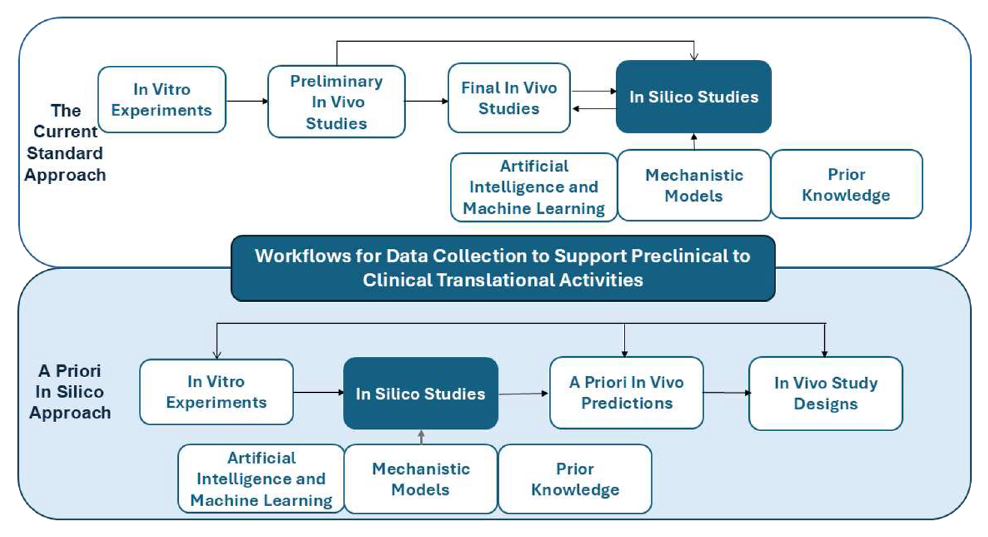
Begin With In Vitro Data
The workflow starts with data from prior knowledge about medicine (disease), physiology (system), and pharmacology. Drug-specific pharmacology knowledge can be gathered using in vitro data, such as, from biochemical assays, cell-based assays, and organ-on-chip platforms.
Build Predictive Models
Tools such as PBPK, quantitative systems pharmacology (QSP), quantitative systems toxicology (QSP), machine learning (ML), or hybrid mechanistic-ML models (explainable/scientific ML) can integrate all available and relevant data to simulate whole-body drug exposure, tissue distribution, and potential risks. This allows species- and/or human-specific predictions of how the drug is expected to behave.
Conduct Reduced, Targeted Animal Studies
Instead of broad exploratory animal testing, a small set of focused in vivo studies can be performed only where uncertainty remains. Such reduced, focused in vivo studies can be designed based on simulations from predictive models. The results from such studies confirms model predictions while significantly reducing total animal use.
Integrate and Refine
Model predictions and confirmatory data can be combined to update the models, guide dose selection, and support decisions for the next. As such, this proposed workflow allow a more efficient, ethical, and human-relevant preclinical strategy.
